7 Film CRYO-ELECTRON MICROSCOPY
CRYO-ELECTRON MICROSCOPY
KRYO-ELEKTRONEN-MIKROSKOPIE
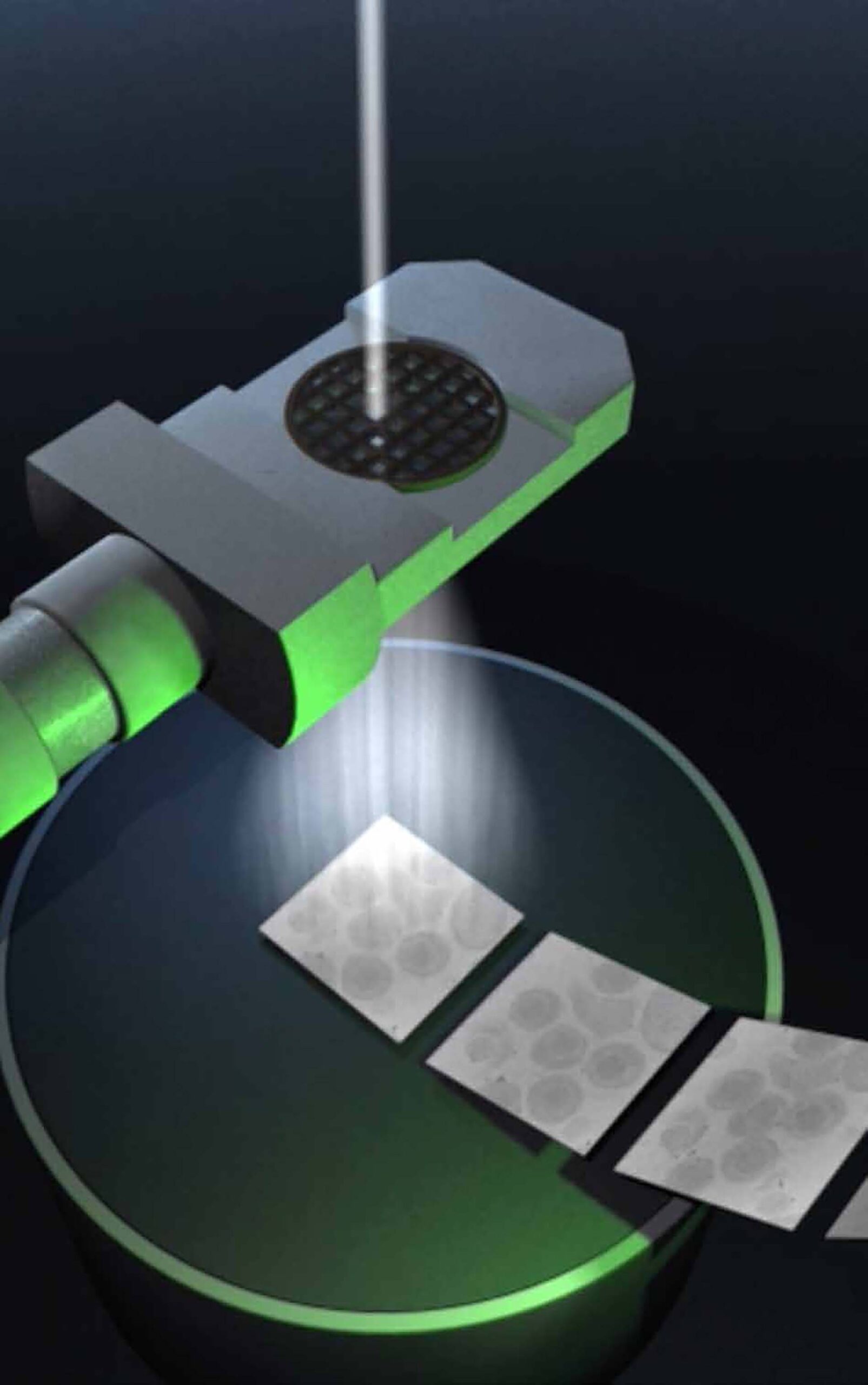
In contrast to X-ray structure analysis, cryo-electron microscopy (cryo-EM) does not require the preparation of protein crystals. Many proteins are very unstable or do not form crystals. The proteins remain in their natural form. In recent years single particle analysis has developed into an efficient method for high-resolution protein structure analysis. It is particularly suitable for large molecular complexes, such as the 26S proteasome, a huge complex composed of 34 different subunits, which is responsible for the targeted degradation of proteins that are no longer needed.
For cryo-EM, proteins are snap-frozen to below -150°C. This freezes the water in the samples to a glass-like state without ice crystals. The samples are then imaged with an electron beam. With the help of computers, high-resolution 3D images are calculated from many different snapshots of individual proteins.
Wolfgang Baumeister is considered the pioneer of cryo-electron tomography, which can be used to study the molecular architecture of cells at high resolution (< 1nm). John Briggs’ department uses this method to study enveloped viruses.